A recent addition to the list of discoveries that stay neglected for long before their full potential is realized – CRISPR – may revolutionize genetic engineering. Two and a half decades after its discovery in the bacterial systems, Clustered Regularly Interspaced Short Palindromic Repeats are now being seen as the tools to edit genomes with precision like never before.
Mechanism
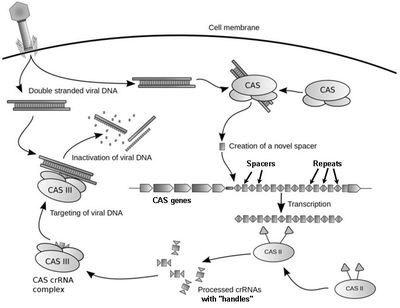
As the name suggests, these are short direct repeats found in most prokaryotes. The CRISPR system confers acquired immunity to prokaryotes. On transfection by viruses, short spacer sequences of the viral genome get incorporated within CRISPR repeats and serve as a memory of the infection. On subsequent infections, these spacers are used to recognize and silence the viral genome in a manner similar to RNA interference.
Certain nuclease or helicase proteins associated with the CRISPR spacers are coded by cas (CRISPR-associated) genes. A single genome may contain more than one CRISPR subtype.
Implications in genetic engineering
The origins of genetic engineering owe much to discovery of restriction enzymes which cut DNA at specific sites. For greater precision, researchers came up with ZFNs (zinc finger nucleases) and TALENs (transcription activator-like effector nucleases) in which the DNA binding domain was engineered to bind to a specific sequence.
Restriction enzymes occur for a vast number of sites, but not for every conceivable sequence. Developing the DNA binding domain for a particular sequence, in the case of ZFNs and TALENs, is a complex process with low success rate. CRISPRs have the great advantage that we just need to ‘write’ the CRISPR-cas system within the genome and it takes care of the precision genome editing by itself.
For synthetic organisms with their genomes written in automated synthesizers rather than tweaked in a conventional lab, CRISPR impart the ability to edit almost any sequence you wish.
A boost to gene therapy?
Famed Harvard geneticist George Church was the first to demonstrate the CRISPR-cas system working in human cells and mouse embryos. In gene therapy, the two major hurdles have been unwanted addition or deletion of nucleotides which may lead to deleterious mutations and the chances of the edit being made somewhere other than the desired site.
Whether this technique is safe enough to make gene therapy a routine clinical procedure is something only time will tell. My bet would be that it would, after a little fine tuning, be safe enough.
Related article:
- CRISPR Cas Powerful Possibilities for Genetic Engineering (nextbigfuture.com)

